Safely Automate Clinical Trial Back Office Tasks


Automating all back office administration for documents, data and communication
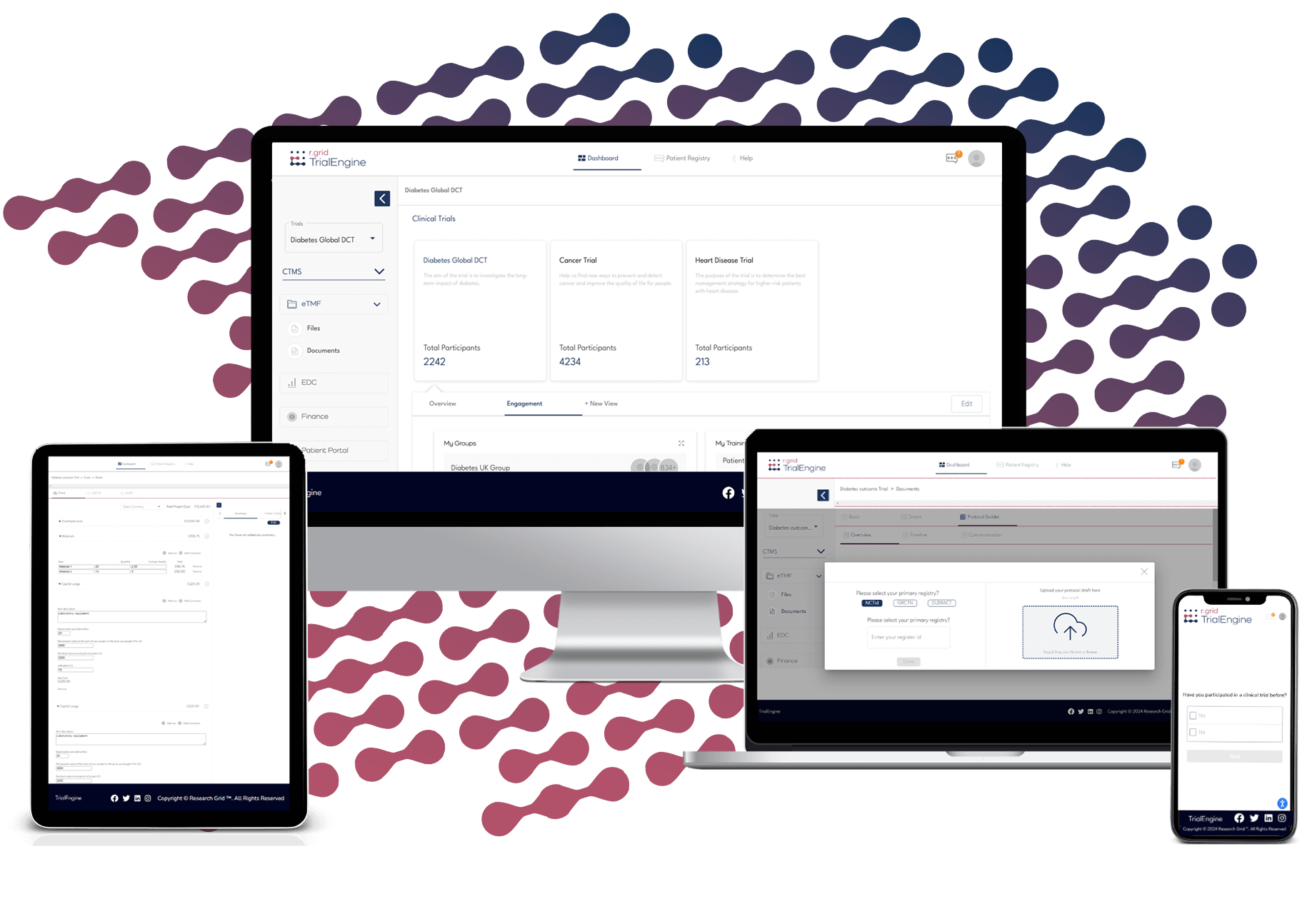
TrialEngine safely accelerates clinical trial operations, improves data quality and increases patient retention
Build and customize dashboards to track trial activity. Switch views, edit widgets, adjust visuals, and download data for quick insights or detailed project reporting.
Create randomization schemes, manage lists, and assign treatments directly within your study workflow. Flexible methodologies and expert guidance support fast, confident setup.
Create, share, and track consent forms with version control, multimedia learning materials to verify understanding, multilingual support, and compliance with HIPAA, GDPR, and global data protection standards.
Site & Sponsor eTMF
Streamline sponsor and site documentation with structured folders, smart templates, change management, and role-based collaboration. Automatically generate key documents like protocols and ICFs to save time and reduce errors.
Streamline sponsor and site documentation with structured folders, smart templates, change management, and role-based collaboration. Automatically generate key documents like protocols and ICFs to save time and reduce errors.
Automate data capture, extraction, and entry for patient visits. Eliminate the need for data reconciliation using real-time migration workflows, quality assurance checks, and automatic patient anonymization.
Collect and manage patient-reported outcomes digitally with real-time surveys, automated extraction, branching logic, and customizable reports. Instantly digitize and anonymize assessments with AI.
Track and manage grant, SoECAT, and AcoRD costs with editable categories, currency settings, hidden fields, equations, and downloadable summaries tailored to your trial budget.
Accelerate innovation, make informed evidence-based decisions, and drive change with integrated data analytics and expert consultancy.
Build relationships directly with community networks, patient advocacy groups, funders, research groups, sites, and other stakeholders through our extensive network.
Centralize quality processes in a single, connected QMS. Manage documents, deviations, CAPAs, and more with real-time visibility. Stay inspection-ready while reducing risk and manual effort.
Connect EHRs, workflow tools, smart devices, and wearables. Search or request apps, configure settings, and use secure APIs to streamline data exchange and automate processes.
Customize the platform with your logo and brand colors. Invite team members with role-based access, manage signatures and privacy settings, and control anonymization.
Receive assistance through live onboarding, searchable guides, and expert technical support providing advanced troubleshooting and integration assistance.
About TrialEngine
TrialEngine is a smart clinical trial automation system (CTAS) that handles the administration, data, and reporting workflows during the trial and post-trial.
It leverages custom AI algorithms and process automation to complete tasks such as protocol development, study documents and data extraction in a matter of minutes.
This contrasts sharply with legacy clinical trial management systems (CTMS) where these tasks can take months or even years. The extremely manual workflows and operations with existing CTMS systems, makes them a major source of bottlenecks and expensive delays.
Security and Compliance
ISO/IEC27001:2022 standard certified
OWASP security tested
Cyber Essentials certified
Registered with the ICO
Computer system validation audited
UKDPA compliant
HIPAA compliant
MHRA compliant
MDR/IVDR compliant
GCP monitoring and data management compliant
NHS Digital Data Security and Protection Toolkit
GDPR compliant